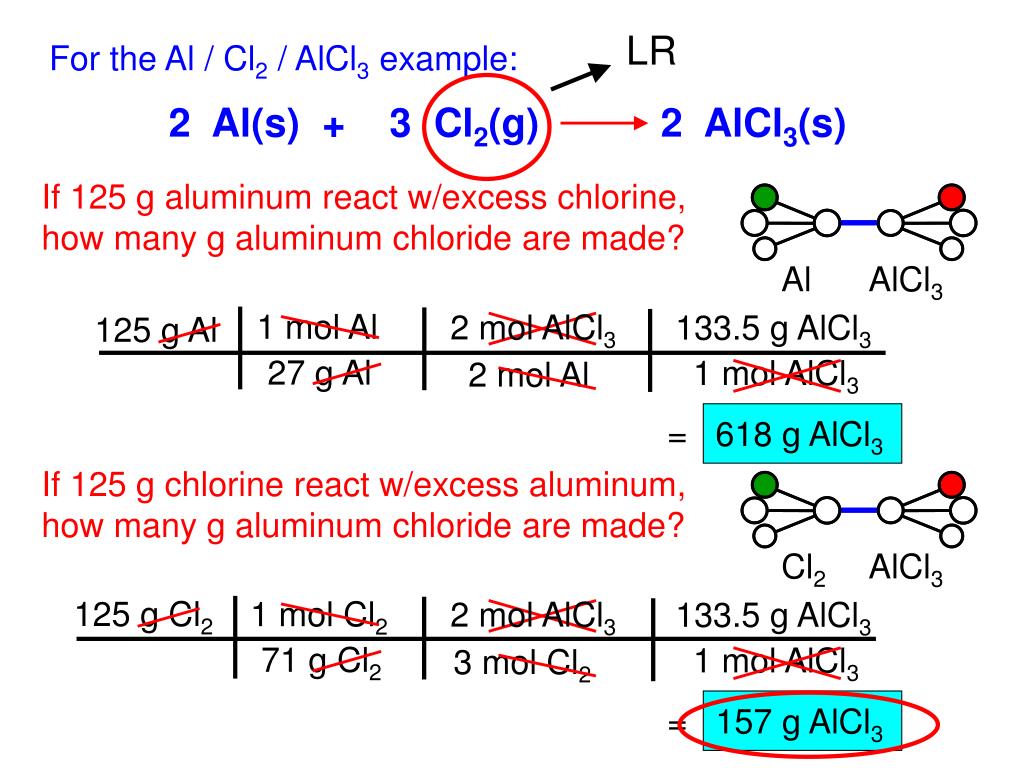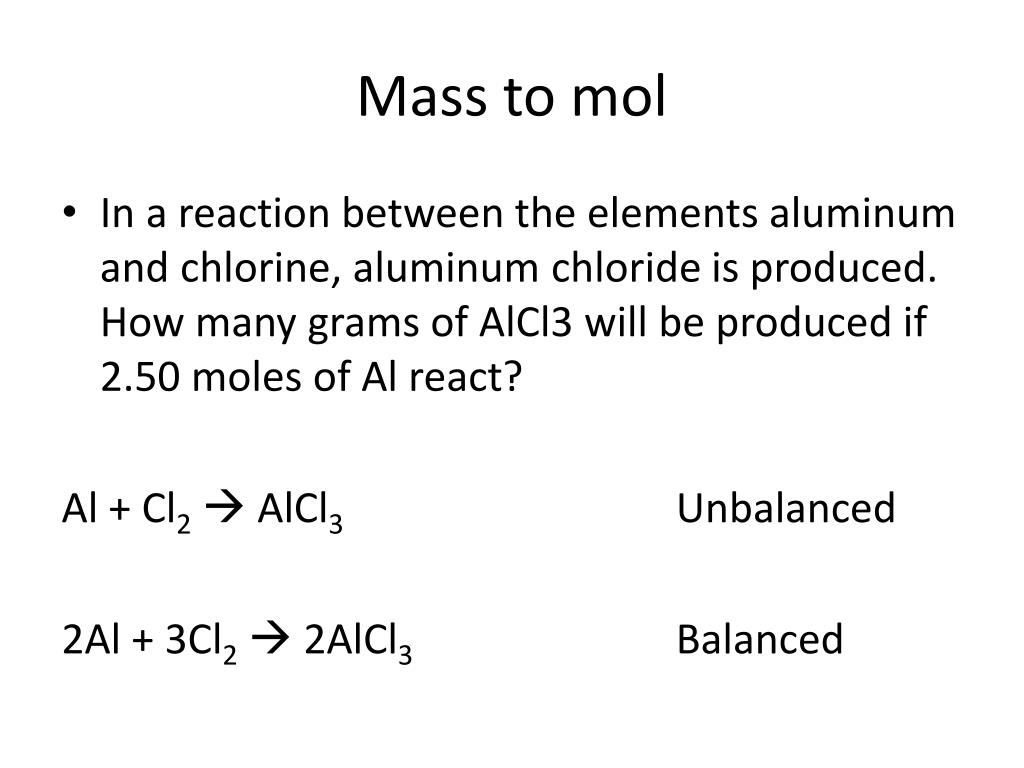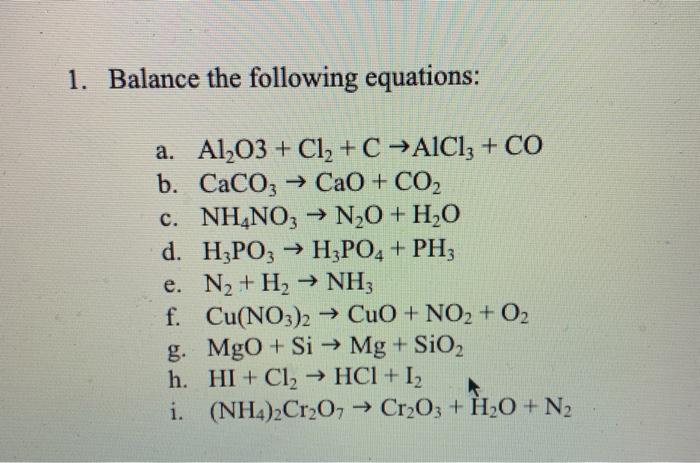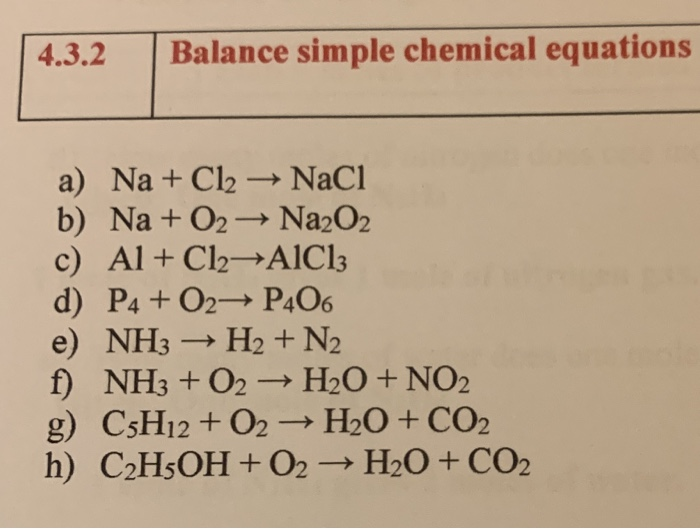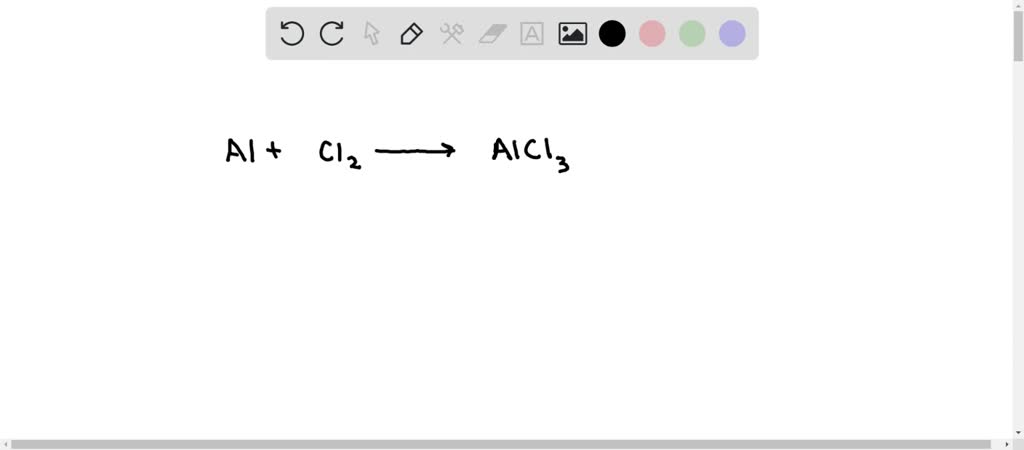
SOLVED: In the following reaction, when the equation is correctly balanced, what is the correct coefficient for aluminum chloride? Al(s) + Cl2(g) â†' AlCl3(s)
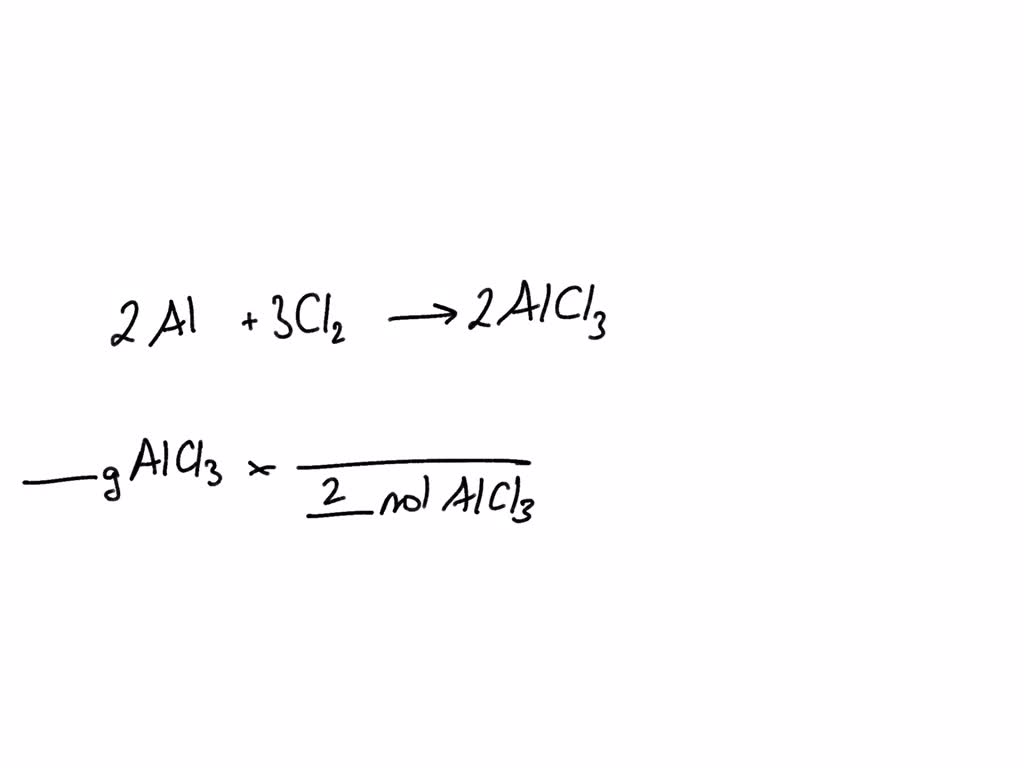
SOLVED: Aluminum reacts with chlorine gas to produce aluminum chloride according to the following equation. Al + Cl2 → AlCl3 Which of the following fractions can be used for the mole ratio
What would the coefficient on Cl2 be when the following reaction is balanced? Al (s) + Cl2 (g) → AlCl3 (s)? - Quora